|
|
Hello {{Contact.FirstName}} {{Contact.LastName}}, |
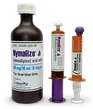
Nymalize: Ready-to-use (RTU) nimodipine reduces time, when every moment counts1Nymalize (6 mg/mL) is well established in medical practices across the United States. Nymalize (6 mg/mL) is a marked improvement over the previous and discontinued formulation (3 mg/mL), with fewer and reduced excipients compared with the previous formulation.2 The 44% reduction of polyethlene glycol 400 (per 60 mg dose), which has laxative properties, may offer the potential for fewer gastrointestinal side effects.2 |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
When you need a liquid nimodipine, choose Nymalize1The RTU nimodipine you trust, brought by a trusted leader in dose-form innovation1 |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Ordering Information |
||||||||||||||||||||||||
|
IMPORTANT SAFETY INFORMATIONIndication and UseNYMALIZE is indicated for the improvement of neurological outcome by reducing the incidence and severity of ischemic deficits in adult patients with subarachnoid hemorrhage (SAH) from ruptured intracranial berry aneurysms regardless of their post-ictus neurological condition (i.e., Hunt and Hess Grades I–V). Important Safety InformationBlood pressure should be carefully monitored during treatment with Nymalize. Nimodipine may increase the blood pressure-lowering–effect of concomitantly administered anti-hypertensives such as diuretics, beta-blockers, ACE inhibitors, angiotensin receptor blockers, other calcium channel blockers, α-adrenergic blockers, PDE5 inhibitors, and α-methyldopa. Patients with cirrhosis are at a higher risk of adverse reactions and should be monitored closely and administered a lower dose. Concomitant use of strong inhibitors of CYP3A4 with nimodipine should generally be avoided because of risk of significant hypotension. These include some macrolide antibiotics (eg, clarithromycin, telithromycin), some HIV protease inhibitors (eg, indinavir, nelfinavir, ritonavir, saquinavir), some HCV protease inhibitors (eg, boceprevir, telaprevir), some azole antimycotics (eg, ketoconazole, itraconazole, posaconazole, voriconazole), conivaptan, delavirdine, and nefazodone. Concomitant use of strong CYP3A4 inducers (eg, carbamazepine, phenobarbital, phenytoin, rifampin, St John’s wort) and nimodipine should generally be avoided, as nimodipine plasma concentration and efficacy may be significantly reduced. Nimodipine plasma concentration can also be increased in the presence of moderate and weak inhibitors of CYP3A4. If nimodipine is concomitantly administered with these drugs, blood pressure should be monitored, and a reduction of the nimodipine dose may be necessary. Grapefruit juice inhibits CYP3A4. Ingestion of grapefruit/grapefruit juice is not recommended while taking nimodipine. Moderate and weak inducers of CYP3A4 may also reduce the efficacy of nimodipine. Patients on these should be closely monitored for lack of effectiveness, and a nimodipine dosage increase may be required. Common Adverse Reactions Most common adverse reactions (incidence ≥1% and ≥1% placebo) were hypotension, headache, nausea, and bradycardia. |
|
The Important Safety Information does not include all the information needed to use NYMALIZE safely and effectively. For additional safety information, please see the full Prescribing Information for NYMALIZE. |
|
To Report SUSPECTED ADVERSE REACTIONS, contact Azurity Pharmaceuticals, Inc. at 1-800-461-7449, or FDA at www.fda.gov/MedWatch or 1-800-FDA-1088. |
|
Warm Regards,
{{User.Name}} |
|
References:
1. NYMALIZE [package insert]. Woburn, MA; Azurity Pharmaceuticals; 2024.
2. Important Safety Consideration: Nimodipine Oral Solution. Evan Schullin, MD; Azurity Pharmaceuticals, Inc. (email communication, January 2025).
|
|
Azurity Pharmaceuticals, Inc. | 8 Cabot Road, Suite 2000, Woburn, MA 01801 The information contained herein, including product information, is intended only for healthcare providers in the United States. Nymalize is a registered trademark of Azurity Pharmaceuticals, Inc. ENFit® is a registered trademark of Global Enteral Device Supplier Association, Inc. © 2025 Azurity Pharmaceuticals, Inc. All Rights Reserved. All trademarks referred to are the property of their respective owners. PP-NYM-US-0946
|